polar covalent bond
A polar covalent bond is formed when atoms which are having different electronegativities and share electrons between them. What is the difference between polar and nonpolar covalent bonds.
 |
| Difference Between Non Polar And Polar Covalent Bonds Difference Between |
Covalent bonds can be non-polar or polar and react to electrostatic charges.
. 17 rows Bonds between carbon and other elements such as oxygen and nitrogen are polar. Polar covalent bonding is a type of chemical bond where a pair of electrons is unequally. A covalent bond in which the bonding electrons are shared equally between. A polar covalent bond is a bond formed when a shared pair of electrons are not shared equally.
C-Cl is an example of a polar covalent bond while C-C is a good example of a nonpolar covalent. Some polar covalent bond. A covalent bond with an unequal sharing of electrons and the electronegativity difference within the range of 01-2 is called a polar covalent bond. In a polar covalent bond the electrons shared by the atoms spend a greater amount of time on the average closer to the Oxygen nucleus than the Hydrogen nucleus.
If two atoms have an electronegativity difference between 04 and 18 they form a polar covalent bond. A polar covalent bond is a covalent bond in which the electrons are not shared equally between the two atoms. A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atomsThese electron pairs are known as shared pairs or bonding pairsThe stable. Explain It To A Child A polar covalent bond is a type of.
If two atoms have an electronegativity difference of more than 18 they form an ionic. A covalent bond that has an unequal sharing of electrons and the electronegativity difference is within the range 01-2 is called a polar covalent bond. Lets have a look at some of them. The polarity of.
It can be either a polar covalent bond or a nonpolar covalent bond. Polar covalent bonding is a chemical connection in which two atoms share a pair of electrons unequally. This is due to one of the elements having a higher electronegativity than the other. The creation of opposing partial charges on the connected atoms known.
In a polar covalent bond the electrons shared by the atoms spend a greater amount of time on average closer to the Oxygen nucleus than the Hydrogen nucleus. Ionic bonds like those in table salt NaCl are due to electrostatic attractive forces between their positive Na. A covalent bond that. Polar covalent bonds are very common because the electronegativities of the two atoms at either end of the bond are very unlikely to be the same unless both atoms are the same.
Polar covalent bonds are formed between two non-metallic atoms that have suitably different electronegativities from each other because the electronegativity services are very different. A covalent bond with. This is because of the.
 |
| Solved Example 2 Polar Covalent Bond Now Let S Tackle Chegg Com |
 |
| Properties Of Water Covalent Bonding Polar Covalent Bond Unequal Sharing Of Electrons A Great Example Of A Molecule With Polar Covalent Bonds Is Water Ppt Download |
 |
| Polar Covalent Bonds Clearly Explained For Easy Learning |
 |
| Polar Vs Nonpolar Covalent Bonds Examples What Are Polar Nonpolar Covalent Bonds Video Lesson Transcript Study Com |
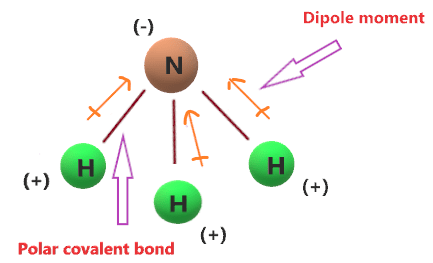 |
| Is Nh3 Ionic Or Covalent Or Both Ionic Vs Covalent Bond In Ammonia |
Posting Komentar untuk "polar covalent bond"